scientific edition of Bauman MSTU
SCIENCE & EDUCATION
Bauman Moscow State Technical University. El № FS 77 - 48211. ISSN 1994-0408
# 03, March 2014
DOI: 10.7463/0314.0705250
Introduction
More than 8 million people in our country suffer from heart failure. About one million of these people die each year [1]. The problem of ventricular assist device creating - a mechanical device used for partial or complete replacement of heart function - is investigated for a long time (according to [2] just in our country since the 1970s). Today plenty of encouraging results are received. There is a number of VAD models which are successfully applied to patients with heart failure. After implantation, patients conduct a way of life that is normal in many respects: they are in the family, often they have an opportunity to work in their former specialty. Some of them live with the device about 8 years [3].According to [4] for 2010 the estimated total number of long-term devices implanted in the United States per year is over 1,700 (the population of the U.S. is 305 million), compared with over 430 per year in Europe (the population of Europe is 731 million). Unfortunately, people who need a heart transplant are much more.
The principle of VAD is that being connected to the left ventricle with one cannula and to the ascending aorta with the other cannula the pump fully or partially replaces the function of the natural heart. This scheme allows the use of VAD in two ways: as a "bridge to transplantation" when the device is used temporarily until the donor heart is found, and a "bridge to recovery", when through the use of VAD the function of the heart muscle is recovered.
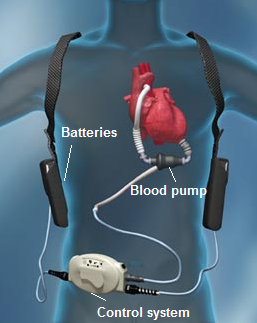
VAD system can be divided into three subsystems: blood pump, power supply system and control system (Fig. 1).
|
Fig. 1. VAD susbsystems [5] |
Each subsystem can be the subject of separate study. Special role in the development of VAD plays medical side of the issue. Successful research and development require interaction with qualified professionals in this field. The development of VAD is a multidisciplinary problem which demands fulfilment of a number of requirements.
One of the most active programs in implantation of long-term systems of artificial circulatory support is guided by German Heart Center in Berlin (DHZB) [3].
The well-known VAD commercial models were created by Berlin Heart (Germany), HeartWare Int. (USA), Thoratec (USA), Abiomed Inc. (USA) etc.
Works devoted to VAD systems can be classified in field of research, according to the above subsystems. Concerning the blood pump - a large number of works is devoted to the study of blood behavior in the pump chamber: to modeling questions [6-24], with an emphasis on red blood cells damage - hemolysis [10, 20, 23, 27-32]. Much less works are devoted to the research of rotor (impeller) dynamics of the blood pump [33-36]. Application of magnetic bearings in VAD and magnetic levitation control tasks of are addressed in [33-39]. Biomedical part of design, associated with the selection and development of biocompatible materials is considered in [26, 32, 40-43]. Features of power supply systems and control of VAD mode work are given in works [26, 44-50].
The integrated approach to VAD development with an emphasis on design phases is presented in the works [7, 51]. In [2] the problem of artificial heart creation in Russia on 2006 is clearly reflected. The most comprehensive systematic review of existing VAD systems with description of specifications is given in the work [54], as well as in [24, 26, 55-57]. Brief review of existing methodologies and approaches to the design of artificial heart (AH) and circulatory support system (CSS) are presented in the works [58, 59]. The problem of creation of mechatronic modules of AH and CSS systems is studied in [60, 61].
With the purpose of further research in the development of VAD system, this work is the study of up to date problem state. The main part of the work consists of three sections. The first section is devoted to the study of blood pump. The second and the third sections are devoted to power supply system and control system respectively. Based on this analysis, the requirements to VAD development are generalized, main issues are underlined and new solutions are proposed in conclusion.
Purpose of investigation.The work is devoted to studying the features of VAD design with the purpose of further research on their optimization and improvement.
1 Blood Pump
Depending on the pump type there are pulsatile, centrifugal and axial VAD [26, 54] (Fig. 2).
|
|
|
a) | b) | c) |
Fig. 2. VAD types: a) pulsatile [62]; b) centrifugal [63]; c) axial [64] | ||
Pulsatile (volume) pumps are Novacor® (Fig. 2, а), Excor®; centrifugal pumps are HeartWare®(Fig. 2, b), Levitronix CentriMag®, Levacor™; axial pumps are DeBackey® (HeartAssist 5) (Fig. 2, c), HeartMate II®, Incor®, Jarvik 2000 etc. [26]. The trends of recent research in this area are associated with the development of the rotary pump models exclusively [14-16, 33-35, 37, 51, 53-56, 65-70]. The operation concept of pulsatile pumps is based on alternate filling of the working chamber with blood and subsequent blood replacement. Disadvantages of this design are the big size of the pump, the fragility of the membrane, the use of valves which can fail when pollution.
VADs are distinguished by location of the pump: inside or outside the body (extracorporeally). An example of an external pump is the model Levitronix CentriMag®, as well as Hemopump®, Abiomed BVS 5000™ etc. A clear disadvantage of such models is limited mobility of the patient, that is why following VAD models are created with the possibility of implantation.
CFD. Simulation of blood flow in the pump chamber is performed using hydrodynamic analysis - Computational Fluid Dynamics (CFD). CFD has been used since the 1960s to analyze fluid flows [51]. With the help of CFD they define the parameters of blood flow, such as: shear stress, pressure drop, velocity distribution, presence of stagnation zones, pressure distribution, hydraulic performance and other. CFD allows to estimate the selected geometry of the blood pump in a short time and at the initial development stage. Different numerical approaches can be applied in CFD:
- finite difference;
- finite volume;
- finite elements;
- smoothed particle hydrodynamics;
- method using of cumulative distribution function.
There are many mathematical modules allowing to carry out simulation of fluid and gas flow: CFX-TASCflow (finite volume), STAR-CD (finite volume), FLUENT (finite volume), SIMPLE algorithm (finite volume), SMAC algorithm (finite difference), Comsol Multiphysics (finite elements), XNS (finite elements) [51]. Modern software packages allow to take into account various effects including turbulence, temperature and thermal phenomena, fluid viscosity as well as to use a model of non-Newtonian fluid in the analysis of the blood dynamics – hemodynamics [7, 72].
The disadvantage of commercial solvers is that the code remains hidden from the user. A developer has no opportunity to look inside the program. In this sense self-developed solvers have the advantage and suggest a more flexible approach to modeling, including optimizing estimated time. Thus, the results given in references [51, 71], are obtained by their own finite element CFD code. But self-developed codes obviously have less user-friendly graphical user interface in comparison with commercial tools [51]. However, it is necessary to distinguish from the commercial finite element software Comsol Multiphysics. In Comsol Multiphysics problem statement is clearly visible when using any module, i.e. for user available are both the equations, describing the process, and the boundary conditions in an explicit form. For example, in ANSYS mathematical statement is hidden from the user behind the element choice. The engineer doesn't see the equations and boundary conditions in that format in which physicists and mathematics got used to see them. In ANSYS the choice of the element means the choice of the equations describing process. In Comsol Multiphysics the choice of the element doesn't oblige you to anything, the equations are set separately at the choice of the module or at setting of their coefficients and constants [73].
Though blood also is non-Newtonian multiphase suspension consisting of plasma, white (leukocytes) and red (erythrocytes) blood cells and platelets [52] simulation of blood flow in large vessels is based on an assumption that blood is homogeneous incompressible Newtonian fluid with constant viscosity. There is a number of justifications of such assumption. Blood is considered homogeneous if it flows through the vessels with a diameter more than two orders of magnitude greater than the size of a red blood cell. This is usually the case for VADs (diameter of human red blood cell ~ 7-8 μm [74]; diameter of abductor cannulas ~ 25 mm; the radial clearance between the screw and the housing is a function of the external screw diameter and the inner diameter of the hub of screw, and for sizes 30 mm and 15 mm it is 0,375-0,75 mm respectively [76]). It is a question of red blood cells because their quantity in usual conditions is three orders of magnitude greater than the quantity of leukocytes and more than one order greater than the quantity of platelets. The maintenance of red blood cells determines the rheological properties of blood. In hemorheology it is known that at shear rates above 100 s-1 blood behaves as a Newtonian fluid (more precisely as pseudo-Newtonian). In VADs the prevalent the shear rates are typically two orders of magnitude higher 100 s-1 [51]. Based on the assumption above the blood flow is described by the basic equations of hydrodynamics – Navier-Stokes equations [6-20, 23, 24, 26-28, 51, 53].
Blood damage.The first problem demanding the decision in VAD development is blood damage – hemolysis. The non-physical blood behavior in pump chamber leads to hemolysis. Under high shear stress, turbulence, strokes at walls of the pump chamber the shell of red blood cells brakes or stretches with pores formation, through which hemoglobin is released. Indicator of hemolysis is one of the most important characteristics of VAD.
One of the opportunities of hemodynamics is hemolysis prediction. For hemolysis prediction Giersiepen suggested an empirical dependence in the form of a model, corresponding to the equation (1), in which is the plasma-free hemoglobin compared to overall amount of hemoglobin in blood,τ is the shear stress (because of non-physiological flow in the pump), t is exposure time and C, a, b are coefficients was proposed

Giersiepen’s model is widely used in studies of the hemolysis phenomenon. Other approaches are given in [23, 51]. In work [7] the author gives full algorithm of hemolysis indicator definition. Blood contact with foreign materials, roughness and coating of flow channel surface, adhesive behavior, as well as stagnation zones promote activation of platelets that leads to thrombosis. Except for detection of stagnation zones during the CFD [7], complexity of the thrombogenesis phenomenon makes it difficult for prediction. One of challenges of VAD development is the analysis and selection of biocompatible materials, as well as determination of requirements to surfaces of flow channel. The first model of platelet aggregation was formulated by Fogelson A.L. in 1992 [19, 20]. The full model consists of the system of coupled differential equations. There are other methods for thrombosis prediction [11, 75, 80], for example, similar hemolysis prediction, using the power transcendental equation [27], however, uniform standard approach for thrombosis prediction does not exist yet. Currently, coating with athrombogenic properties are prepared essentially by using heparin - natural sulfated polysaccharide. Such materials include, for example, one of the most successful commercial products Сarmeda [77]. Additionally, bioinert polymer coating based on poly-p-xylylene and its derivatives are also used [26]. To the surfaces contacting with blood belongs not only flow channel of the pump, but also adductor and abductor cannulas.
Miniaturization. Rotor speed, significantly affecting the indicator of hemolysis, relates to the size of the pump. Capacity of existing commercial VAD models (HeartWare®, Levitronix CentriMag®, HeartMate III®, DeBackey®, Incor®, Heart Mate II®) designed for long-term use is in average 9 L·min-1. Volume flow of Impella® and its modifications changes from 2,5-5 L·min-1 as Impella® is a short-term mechanical system of heart function support. For 2,5 L·min-1 and a diameter of the pumping chamber equal to 4,2 mm rotation speed is 51,000 rpm [78]. At the same time Incor® at flow rate 10 L·min -1 and the diameter of the pumping chamber 30 mm has maximum rotor speed equal to 10,000 rpm. It should be noted that axial pumps are characterized by bigger rotor speeds, than centrifugal ones [26]. Of course, the smaller the dimensions of the pump are less feelable it is in the body.
Implantation configuration.Size, weight and configuration of the blood pump are choosen not only for reasons of blood safety and effectiveness of the device, but also for reasons of anatomical preferences. VADs must be easily implanted. Implantation of the mechanical heart support device should be safe for the patient and comfortable to the surgeon. General availability of such operation will be defined not only by cost, but also by complexity and risk level. Almost all used VAD commercial models are implanted by sternotomy. Difference make CircuLite® Synergy – the hybrid of the centrifugal and axial pump (the diagonal pump) and Impella®. Input cannula of the CircuLite® Synergy is connected to the left atrium and the output cannula is connected to the right subclavian artery. CircuLite® Synergy was appeared on the market recently. The European certification by data [79] was planned for 2012. Impella® is implanted endovaskular through a femoral artery which makes such operation close to usual procedure [69, 78].
Reliability. Severity of complications which can develop in case of VAD failure puts rigid requirements for reliability and nonfailure work throughout all the operating life of the device. The key moment of pump part analysis is the analysis of rotordynamics.
In previous VADs models hydrodynamic jewelled tilling bearings, immersed in blood, perform as a support [26, 32]. In new generations of VADs they actively began to apply magnetic bearings. In ball bearings blood can't be used as lubricants, because high shear stress in a small ball-race clearance damages red blood cells. Application of other types of lubricants provokes platelets activation which leads to the thrombogenesis. Centrifugal pumps, as a rule, are used with hydrodynamic bearings with the minimum gap in which the main destruction of blood red cells can happen due to significant shear stress [80]. In addition, widespread industrial experience with hydrodynamic bearings has high bearing failure rates [33].
Magnetic suspension has the advantages of non-contact support: high durability due to the absence of friction and a bigger clearance, allowing the rotor to rotate at higher speeds with the uprise of not-too-high shear stress. The use of magnetic bearings in VADs reduces indicators of hemolysis and thrombosis and increases lifetime of the device, but it requires more rigid control. The PID control of the VAD magnetic suspension is spread [34, 36, 38, 81], as well as sliding mode control [34], PI control [70, 82], optimal [82, 83] and suboptimal control [39]. Magnetic levitated rotor is one of the examples of nonlinear systems, intensive studies of which are dedicated to finding optimal stable control.
2 Power supply system
The power source is necessary for functioning of VADs.
Infection.Modern VAD models suggest several options of power supply systems. The most widespread way of energy transmission is through the wires, which are passing through patient skin and connecting pump part to a network or batteries, being outside. Power supply from the network doesn't provide a sufficient level of mobility for normal activity for the patient. In this sense the use of accumulators, which are set in a backpack or on a belt is much more convenient. But such option of power supply in principle is dangerous, because the place of wires entrance is a source of infections, which almost in 100 percent of cases getting into the human organism lead to a fatal case.
The second option - wireless power transmission. In addition to medical features associated with the exception of infection, non-contact power transmission provides more freedom of movement for the patient. Moreover it allows power transmission from an implanted battery or using wireless power transmission channel [44-50].
For power transmission at a distance, there are two technologies: TETS (a transcutaneous energy transformer system) and FREE-D (free-range resonant electrical energy delivery). In both cases coils wound of litzendraht are used [84].
VADs power supply from implanted batteries technology is currently used only in conjunction with power supply through the wireless channel of energy transmission as accumulators, because modern implanted batteries don’t have sufficient capacity for long-term work without replacement, and replacement is made by an invasive way. For a continuous power supply of VADs it is necessary to have a source with power up to 10 Watts (a nominal mode, with the flow rate 10 L·min -1) [26]. Currently, by Jarvik Heart company input of power and control wires through the mastoid bone of the skull was proposed (VAD model – Jarvik 2000) [55]. This method solves the infection problem rather effectively, but possible problems of the input unit survival have to be noted, as well as the need to lay wires of considerable length from the patient's head to supply elements located on the belt.
The following requirements are imposed to power supplies: compactness, safety, biocompatibility (for implanted supplies), reliability, long period of work [26]. The problem of VAD power supply should be solved globally because exactly this problem raises a question of normal activity and mobility of the patient.
3 Control of VADs work
Along with biomedical and design-technology tasks there are problems of effective control of VADs work.
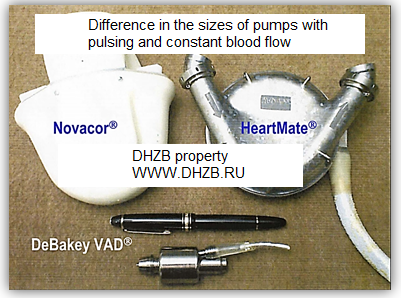
Cardiac cycle consists of two phases: systole and diastole. Unsteady flow of blood with some approximation is taken periodic in time. From these considerations, the first models of VADs are of pulsatile type, for example, Novacor®, Abiomed BVS 5000®, HeartMate® and create pulsating flow [7, 9,26, 54, 85]. Big sizes of pulsatile pumps (Fig. 3) resulted in need of VADs creation with constant blood flow.
|
Fig. 3. Pumps with pulsating flow Novacor®, HeartMate®. Pumps with constant flow: DeBackey® [3] |
All used pump models possess the flow rate, allowing to provide different operating modes of heart (high activity, normal mode, dream period). The necessary quantity of high-speed modes and smooth regulation of rotor rotation speed is provided. For this purpose for the control system [26]:
- the main parameters determining heart intensity of the heart are established;
- parameters for continuous monitoring of the organism and heart operation control are chosen;
- system of parameters which will provide individual settings of control system and satisfy specific features of an organism; these parameters must be controlled periodically with a significant time period, in order to monitor their changes over time.
Optimal management of VADs operation is vital for the patient.
Summary and Conclusions
The development of VAD system is a complex multidisciplinary technical and biomedical task. Considered features of VADs development in total allow to formulate the main requirements imposed to developers [7, 9, 14-16, 26, 32, 36, 86]:
- numerical simulation of hemodynamics (hemolysis);
- taking into account hemocompatibility (thrombosis);
- maximum miniaturization and selection of new materials;
- implantation configuration (ease of surgery);
- ensuring reliability of all the subsystems (long mechanical life);
- protection/prevention of infection.
All these requirements are anyway connected with each other or follow one from another. Finally, all of them are aimed to achievement of the only goal – improvement of life quality. Thus the cost of systems has to be adequate [51, 87]. Therefore the provided list can be expanded by two more points:
- quality of life;
- costs.
Currently VADs are far from widespread application owing to their imperfection: blood damage, existance of percutaneous power and control wires, heavy energy sources, which need to be carried constantly with oneself and extremely high cost. However, modern technologies open great opportunities for improvement in this area.
One of these achievements is 3D-printing. Rapid prototyping allows to create a prototype from almost any material (steel, titanium and other alloys, plastic, glass, wood, etc.) quickly. It reduces considerably production time: the model is printed in few hours, is tested, in case of need is refined – so, the working cycle is repeated until development is brought to perfection. In addition, it significantly reduces development costs due to lack of need for technical production and due to early detection of the development errors [88, 89].
Advantages of magnetic bearings allow to achieve smaller blood damage. The problem of optimal stable control can be solved with the help of synergetic control theory which is based on principles and methods of directed self-organization, allowing to take into account nonlinear properties of the object [90]. In the last decades the synergetic method of nonlinear objects control systems synthesis became widespread due to its advantages [90-96].
Application of new fluid flow models, development of an optimal blood pump design, application of new biocompatible materials and wireless power transmission realization can also contribute to improvement of current results.
References
- Okolo milliona rossijan ezhegodno umiraet ot serdechnoj nedostatochnosti [One million people die each year from heart failure]. RIA Novosti: website, 17 May 2013. Available at: http://ria.ru/society/20130517/937886634.html , accessed 20.01.2014. (in Russian).
- Itkin G.P. [Artificial heart development problem: the current status]. Problemy upravlenij, 2006, no. 3, pp. 57-67. (in Russian).
- Nemeckij kardiologicheskij centr v Berline [Deutsches Herzzentrum Berlin]; website. Available at: http://www.dhzb.ru/ , accessed 17.01.2014. (in Russian).
- Birks E.J. The Comparative Use of Ventricular Assist Device: Differences between Europe and the United States. Texas Heart Institute Journal, 2010, vol. 37, no. 5, pp. 565-567.
- Thoratec HeartMate II® LVAD. Available at: http://www.mylvad.com/lvad-devices/heartmate-ii-lvad , accessed 10.02.2014.
- Bokerija L.A., Nikolaev D.A., Fadeev A.A. [Prosthetic heart valves supporting involute blood flow structure]. Klinicheskaya fiziologiya krovoobrashcheniya, 2009, no. 2, pp. 47-51. (in Russian)
- Morozov V.V., Zhdanov A.V., Shmygov E.I., etc. Sintez iskusstvennyh zheludochkov serdca s zadannymi gemodinamicheskimi harakteristikam [Synthesis of artificial heart ventricles with specified hemodynamic characteristics]. Vladimir, VlSU Publ., 2007. 180 p. (in Russian)
- Apel J., Neudel F., Reul H. Computational Fluid Dynamics and Experimental Validation of a Microaxial Blood Pump. ASAIO Journal, 2001, vol. 47, pp. 552-558.
- Behbahani M., Behr M., Hormes M., Steinseifer U., Arora D., Coronado O., Pasqualia M. Review of Computational Fluid Dynamics Analysis of Blood Pumps. European Journal of Applied Mathematics, 2009, vol. 20, pp. 363-397.
- Bartesaghi S., Colombo G. Embedded CFD Simulation for Blood Flow. Computer-Aided Design and Applications, 2013, vol. 10, no. 4, pp. 685-699.
- Sousa L., Castro C., Antonio C., Chaves R. Computational Techniques and Validation of Blood Flow Simulation. WSEAS Transactions on Biology and Biomedicine, 2011, vol. 8, iss. 4, pp. 145-155.
- Deparis S. HPC for Blood Flow Simulations: Advancements and Challenges. Ecole Polytechnique Federale De Lausanne, 2012. 40 p.
- Behnia M., Ohta M., Srinivas K., Nakayama T. Fluid Dynamics of Blood Flow – Modelling and Simulation. Proc. of the 13th Annual Scientic Meeting and Workshops, 06-11 February 2010, Auckland, New Zealand, 2010. 75 p.
- Behbahani M. Finite Element Simulation of Blood Flow. RWTH Aachen, 2006. 34 p.
- Behr M., Arora D., Nosé Y., Motomura T. Performance Analysis of Ventricular Assist Devices Using Finite Element Flow Simulation. International Journal for Numerical Methods in Fluids, 2004, vol. 46, no. 12, pp. 1201-1210. DOI: 10.1002/fld.796
- Fraser K.H., Taskin M.E., Griffith B.P., Wu Z.J. The use of computational fluid dynamics in the development of ventricular assist devices. Medical Engineering and Physics, 2011, vol. 33, no. 3, pp. 263-80.
- Quarteroni A., Formaggia L. Mathematical Modelling and Numerical Simulation of the Cardiovascular System. Ecole Polytechnique Federale De Lausanne, 2002. 103 p.
- Veneziani A., Vergara C. Flow rate defective boundary conditions in hemodynamics simulations. International Journal for Numerical Methods in Fluids, 2005, vol. 47, pp. 803-816. DOI: 10.1002/fld.843
- Vignon-Clementel I.E. A Coupled Multidomain Method for Computational Modeling of Blood Flow. Stanford University, 2006. 207 p.
- Yang X.S., Lewis R.W., Zhang H.Y. Finite Element Analysis of Fogelson's Model for Platelet Aggregation. Proc. of the European Congress on Computational Methods in Applied Sciences and Engineering, 2004. 5 p.
- Zhang L., Jia Y., Zhang W., Wang Y., Zhao Q. Numerical Simulation Investigation on Flow Field of Axial Blood Pump. In: Advances in Computer Science and Engineering. Springer Berlin Heidelberg, 2012, pp. 223-229. (Ser. Advances in Intelligent and Soft Computing; vol. 141.). DOI: 10.1007/978-3-642-27948-5_31
- Beljaev L.V., Ivanchenko A.B., Zhdanov A.V. [Hemodynamic aspects of design of the systems of auxiliary blood circulation and artificial heart on base mechatronic modules]. Sovremennye problemy nauki i obrazovanija - Modern problems of science and education, 2013, no. 3. Available at: http://www.science-education.ru/en/109-9195 , accessed 01.02.2014. (in Russian)
- Behr M., Arora D., Coronado O., Pasquali M. Models and Finite Element Techniques for Blood Flow Simulation. International Journal for Computational Fluid Dynamics, 2006, vol. 20, pp. 175-181.
- Doyle M.G. Simulation of Blood Flow in a Ventricular Assist Device with Fluid-Structure Interaction Effects: Degree of Master of Applied Science. University of Ottawa, 2004. 116 p.
- Bonnemain J., Deparis S., Quarteroni A. Connecting ventricular assist devices to the aorta: a numerical mode: MATHICSE Technical Report. Ecole Polytechnique Federale De Lausanne, 2012. 19 p.
- Koroteev A.V., et al. Razrabotka kompleksa biotehnicheskih sistem "iskusstvennoe serdce" dlja zameshhenija/podderzhanija funkcii serdca. (Jetap 1: Vybor napravlenija issledovanij. Teoreticheskie i jeksperimental'nye issledovanija postavlennyh pered NIR zadach): otchet o NIR [Development of complex biotechnological systems "artificial heart" for replacement/support of heart functions. (Stage 1: Selecting the direction of research. Theoretical and experimental research): research report]. Moscow, NRC "Kurchatov Institute", 2013. 80p. (in Russian, unpublished).
- Alemu Y., Bluestein D. Flow Induced Platelet Activation and Damage Accumulation in a Mechanical Heart Valve: Numerical Studies. Artificial Organs, 2007, vol. 31, pp. 677-688. DOI: 10.1111/j.1525-1594.2007.00446.x
- Arora D., Behr M., Pasquali M. Blood damage measures for ventricular assist device modeling. In: Mammoli A.A., Brebbia C.F., eds. Moving Boundaries VII: Computational Modelling of Free and Moving Boundary Problems. WIT Press, Southampton, UK, 2003, pp. 129-138.
- Goodman P.D., Barlow E.T., Crapo P.M., Mohammad S.F., Solen K.A. Computational Model of Device-Induced Thrombosis and Thromboembolism. Annals of Biomedical Engineering, 2005, vol. 33, no. 6, pp. 780-797. DOI: 10.1007/s10439-005-2951-z
- Sorensen E.N., Burgreen G.W., Wagner W.R., Antaki J.F. Computational Simulation of Platelet Deposition and Activation: 1. Model Development and Properties. Annals of Biomedical Engineering, 1999, vol. 27, no. 4, pp. 436-448. DOI: 10.1114/1.200
- Sorensen E.N., Burgreen G.W., Wagner W.R., Antaki J.F. Computational Simulation of Platelet Deposition and Activation: 2. Results for Poiseuille Flow over Collagen. Annals of Biomedical Engineering, 1999, vol. 27, no. 4, pp. 449-458. DOI: 10.1114/1.201
- Rau G. Research Report 2001/2002. Institute for Biomedical Technologies, Aachen University, 2002. 101 p.
- Allaire P.E., Kim H.C., Maslen E.H., Bearnson G.B., Olsen D.B. Design of a Magnetic Bearing-Supported Prototype Centrifugal Artificial Heart Pump©. Tribology Transactions, 1996, vol. 39, no. 3, pp. 663-669.
- Gomez A.D. Control of a magnetically levitated ventricular assist device: Degree of Master of Science in Mechanical Engineering. Rochester Institute of Technology, 2009. 140 p.
- Noh M.D., Antaki J.F., Ricci M., Gardiner J., Paden D., Wu J., Prem E., Borovetz H., Paden B.E. Magnetic Design for the PediaFlow Ventricular Assist Device. Artificial Organs, 2008, vol. 32, no. 2, pp. 127-135. DOI: 10.1111/j.1525-1594.2007.00501.x
- Greatrex N.A. Design of Physiological Control and Magnetic Levitation Systems for a Total Artificial Heart: Degree of Doctor of Philosophy. Queensland University of Technology, Faculty of Science and Engineering, 2013. 319 p.
- Antunes P. Magnetic Suspension of the Rotor of a Ventricular Assistance Device of Mixed Flow Type – Hall Sensor for Rotor Position Measurement – Use of Compensator. ABCM Symposium Series in Mechatronics. Vol. 5: Section 8 - Sensors & Actuators, 2012, pp. 1249-1256.
- Cheng S., Olles M.W., Burger A.F., Day S.W. Optimization of a Hybrid Magnetic Bearing for a Magnetically Levitated Blood Pump via 3-D FEA. Mechatronics (Oxf), 2011, vol. 21, no. 7, pp. 1163-1169. DOI: 10.1016/j.mechatronics.2011.07.010
- Barbaraci G., Mariotti G.V. Sub-Optimal Control Law for Active Magnetic Bearings Suspension. Journal of Control Engineering and Technology (JCET), 2012, vol. 2, no. 1, pp. 1-10.
- Bosiers M., Deloose K., Verbist J, Schroe H., Lauwers G., Lansink W., Peeters P. Heparin-bonded expanded polytetraflouroethylene vascular graft for femoropoliteal and femorocrural bypass grafting: 1-years results. Journal of Vascular Surgery, 2006, vol. 43, no. 2, pp. 313-319.
- Von Segesser L.K. Safety and efficacy of heparin-bonded surfaces in cardiopulmonary bypass. Thoracic and Cardiovascular Surgery, 2001, vol. 121, pp. 200-201.
- Gorman R.C., Ziats N., Rao A.K., Gikakis N., Sun L., Khan M.M., Stenach N., Sapatnekar S., Chouhan V., Gorman J.H., Niewiarowski S., Colman R.W., Anderson J.M., Edmunds .LH. Jr. Surface-bound heparin fails to reduce thrombin formation during clinical cardiopulmonary bypass. Journal of Thoracic and cardiovascular surgery, 1996, vol. 111, pp. 1-12.
- Hench L.L., Jones J.R. Biomaterials, Artificial Organs and Tissue Engineering. Woodhead Publishing Limited, 2005. 287 p. (Russ. ed.: Hench L., Jones D. Biomaterialy, iskusstvennye organy i inzhiniring tkanej. Moscow, Tehnosfera, 2007. 304 p.).
- Kurs A., Moffat R., Soljaĉić M. Simultaneous mid-range power transfer to multiple devices. Appl. Phys. Lett., 2010, vol. 96, p. 044102. DOI: 10.1063/1.3284651
- Kürschner D., Rathge C. Contactless energy transmission systems with improved coil positioning flexibility for high power applications. Proc. of the 39th IEEE Annual Power Electronics Specialists Conference (PESC '08), Rhodes, Greece, 2008, pp. 4326-4332. DOI: 10.1109/PESC.2008.4592639
- Ricketts D.S., Chabalko M.J., Hillenius A. Experimental demonstration of the equivalence of inductive and strongly coupled magnetic resonance wireless power transfer. Appl. Phys. Lett., 2013, vol. 102, p. 053904. DOI: 10.1063/1.4788748
- Lee W.S. Uniform magnetic field distribution of spatially structured resonant coil for wireless power transfer. Appl. Phys. Lett., 2012, vol. 100, p. 214105. DOI: 10.1063/1.4719585
- Kurs A., Karalis A., Moffatt R., Joannopoulos J.D., Fisher P., Soljacic M. Wireless power transfer via strongly coupled magnetic resonances. Science, 2007, vol. 317, no. 5834, pp. 83-86.
- Bonde P., Sample A.P., Waters B., Cooper E., Toyoda Y., Kormos R.L., Smith J.R. Wireless Power for Ventricular Assist Devices: Innovation with the Free-Range Resonant Electrical Energy Delivery System (FREE-D) for Mechanical Circulatory Assist. AATS 91st Annual Meeting, May 7 - 11, 2011, Pennsylvania Convention Center, Philadelphia, PA, 2011.
- Waters H.B., Sample A.P., Bonde P., Smith J.R. Powering a Ventricular Assist Device (VAD) With the Free-Range Resonant Electrical Energy Delivery (FREE-D) System. Proc. of the IEEE, 2012, vol. 100, no. 1, pp. 138-149. DOI: 10.1109/JPROC.2011.2165309
- Finocchiaro T., Heinke S., Behbahani M., Leßmann M., Laumen M., Steinseifer U., Schmitz-Rode T., Leonhardt S., Behr M., Hamayer K. Methods of design, simulation, and control for the development of new VAD/TAH concepts. Biomedizinische Technik. Berlin, 2009, vol. 54, pp. 269-281.
- How T.V. Advances in hemodynamics and hemorheology: vol. 1. Connecticut, London, JAI Press Inc., 1996. 449 p.
- Nobile F. Numerical Approximation of Fluid-Structure Interaction Problems with Application to Hemodynamics. Ph.D. thesis. Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland, 2001.
- Reul H.M., Akdis M. Blood pumps for circulatory support. Perfusion, 2000, vol. 15, pp. 295-311.
- John R. Current Axial-Flow Devices – the HeartMate II® and Jarvik 2000 Left Ventricular Assist Devices. Seminars in Thoracic and Cardiovascular Surgery, 2008, vol. 20, no. 3, pp. 264-272. DOI: 10.1053/j.semtcvs.2008.08.001
- Timms D. A review of clinical ventricular assist devices. Medical Engineering and Physics, 2011, vol. 33, no. 9, pp. 1041-1047.
- Circulatory and Ventricular Assist Devices (VAD). Available at: http://www.med.umich.edu/cardiac-surgery/patient/adult/ccs/vad.shtml , accessed 10.02.2014.
- Beljaev L.V., Zhdanov A.V. [The features of modern approaches to design of pulsative type systems of artificial heart and auxiliary blood circulation with cals-technologies application]. Sovremennye problemy nauki i obrazovanija - Modern problems of science and education, 2013, no. 6. Available at: http://www.science-education.ru/en/113-11654 , accessed 01.02.2014. (in Russian).
- Zhdanov A.V., Belyaev L.V., Kulikov S.V., Kilasev N.B., Drobyshev A.A. [The modern approach to designing of the artificial ventricles of heart based on CALS-technologies]. Sovremennye problemy nauki i obrazovanija - Modern problems of science and education, 2012, no. 5. Available at: http://www.science-education.ru/en/105-7077 , accessed 01.02.2014. (in Russian).
- Zhdanov A.V., Belyaev L.V., Shevchenko A.P. [Geometrical synthesis of the space cam mechanism of mechatronic unite for auxiliary blood circulation systems]. Sovremennye problemy nauki i obrazovanija - Modern problems of science and education, 2013, no. 4. Available at: http://www.science-education.ru/en/110-9708 , accessed 01.02.2014. (in Russian).
- Volkova I.V., Zhdanov A.V. [Вesign actuator pump systems assist circulation and artificial heart]. Nauchno-tekhnicheskiy vestnik Povolzh'ya, 2011, no. 2, pp. 59-63. (in Russian).
- Heart transplant reunion party celebrates lifesaving milestone. Available at: http://med.stanford.edu/patient_care/spotlight/archive/lvad.html , accessed 28.03.2013.
- HeartWare® Ventricular Assist System: System Overview Hands-on Practicum. HeartWare, Inc., 2012. 70 p.
- NASA. Blood Pump. Available at: http://www.nasa.gov/audience/foreducators/informal/features/F_Blood_Pump.html , accessed 28.03.2013.
- DeBakey M.E. A miniature implantable axial flow ventricular assist device. Annual Thoracic Surgery, 1999, vol. 68, pp. 637-640.
- Pagani F.D. Continuous-Flow Rotary Left Ventricular Assist Devices "3rd Generation" Design. Thoracic and Cardiovascular Surgery, 2008, vol. 20, pp. 255-263.
- Frazier O.H., Khalil H.A., Benkowski R.J. Optimization of axial-pump pressure sensitivity for a continuous-flow rotary left artificial heart. Journal of Heart Lung Transplant, 2010, vol. 29, pp. 687-691.
- Lim Tau Meng, Cheng Shanbao. Development of Hybrid Magnetic Bearings System for Axial-Flow Blood Pump. In: Alan H. S. Chan, Sio-Iong Ao, eds. Advances in Industrial Engineering and Operations Research. Springer US, 2008, pp. 391-400. DOI: 10.1007/978-0-387-74905-1_28
- Weber D.M., Raess D.H., Henriques J.P.S., Siess T. Principles of Impella cardiac support: The evolution of cardiac support technology toward the ideal assist device. Cardiac Intervention Today, 2009, suppl. Principles of Hemodynamics, pp. 3-16. Available at: http://citoday.com/pdfs/0909_supp.pdf , accessed 01.02.2014.
- Leão T., Bock E., Uebelhart B., Fonseca J., Silva B., Leme J., Silva C., Andrade A. Study of speed control of the implantable centrifugal blood pump to avoid aortic valve stenosis. Proc. of the 22nd International Congress of Mechanical Engineering (COBEM 2013), 2013, pp. 6133-6138. Available at: http://cobem2013.com.br/cd/PDF/1406.pdf , accessed 01.02.2014.
- Milenin A., Kopernik M. Comparative analysis of ventricular assist devices (POLVAD and POLVAD_EXT) based on multiscale FEM model. Acta of Bioengineering and Biomechanics, 2011, vol. 13, no. 2, pp. 13-23.
- Baskurt O.K., Hardeman M.R., Rampling M.W., Meiselman H.J., eds. Handbook of Hemorheology and Hemodynamics. IOS Press, 2007. 469 p.
- ANSYS vs Comsol Multiphysics. Available at: http://dolivanov.ru/node/152 , accessed 10.02.2014. (in Russian).
- Robertson A.M., Sequeria A., Kameneva M. Hemorheology. Hemodynamical Flows. Modeling, Analysis and Simulation. Oberwolfach Seminars, 2008, vol. 37, pp. 63-120.
- Goodman P.D., Barlow E.T., Crapo P.M., Mohammad S.F., Solen K.A. Computational Model of Device-Induced Thrombosis and Thromboembolism. Annals of Biomedical Engineering, 2005, vol. 33, iss. 6, pp. 780-797. DOI: 10.1007/s10439-005-2951-z
- Ovsjannikov B.V., Selifonov V.S., Chervakov V.V. Raschet i proektirovanie shnekocentrobezhnogo nasosa [Calculation and design of the screw-centrifugal pump]. Moscow, MAI Publ., 1995. 72 p. (in Russian).
- Carmeda® BioActive Surface: Broshure. Medtronic, Inc., USA, 2010. 12 p.
- Impella 2.5 with the Impella Console: Circulatory Support System: Instructions for Use and Clinical Reference Manual: Abiomed Europe Gmbh. Document No. 0046-9027 Rev. A., 2010. 140 p.
- Agarwal S., High K.M. Newer-generation ventricular assist devices. Best Practice and Research Clinical Anaesthesiology, 2012, vol. 26, pp. 117-130.
- Wu J., Paden B.E., Borovetz H.S., Antaki J.F. Computational Fluid Dynamics Analysis of Blade Tip Clearance on Hemodynаmic Performance and Blood Damage in a Centrifugal Ventricular Assist Device. Artificial Organs, 2010, vol. 34, iss. 5, pp. 402-411. DOI: 10.1111/j.1525-1594.2009.00875.x
- Dongsheng Z. Development of an enclosed-impeller ventricular assist device using self-bearing motor: Degree of Doctor of Philosophy. School of Mechanical and Aerospace Engineering, 2008. 179 p.
- Wu Y., Allaire P., Tao G., Olsen D. Modeling, Estimation and Control of Cardiovascular Systems with a Left Ventricular Assist Device. Proc. of the 2005 American Control Conference. Vol. 6, Portland, Oregon, 2005, pp. 3841-3846.
- Tavoularis S., Ahmed N.U., Madrane A., Vaillancourt R. Towards Optimal Control of Blood Flow in Artificial Hearts. Cardiovascular Engineering, 2003, vol. 8, no. 1-2, pp. 20-31.
- Sherman C., Daly B., Dasse K., Clay W., Szycher M., Handrahan J., Schuder J., Hopkins R., Poirier V., Haudenschild C. Research and Development: Systems for Transmitting Energy through Intact Skin. Final Technical Report No. N01-HV-0-2903-4. National Heart, Lung and Blood Institute, 1984. 211 p.
- Barteld K.P. Implantable electromechanical displacement blood pumps: systematic design and validation methods: Dissertation. Fakultät für Maschinenwesen der Rheinisch-Westfälischen Technischen Hochschule Aachen, 2007. 157 p.
- Reesink K.D. Modelling and Control Aspects of Left Ventricular Assist. Technische Universiteit Eindhoven, 2002. 109 p.
- Sozdannoe s uchastiem MIJeTa otechestvennoe "iskusstvennoe serdce" vpervye implantirovali cheloveku [Created with the participation of NRU MIET "artificial heart" has been implanted]. Available at: http://imm.org.ua/mednews/Sozdannoe-s-uchastiem-MIETa-otechestvennoe-iskusstvennoe-serdce-vpervie-implantirovali-cheloveku.html ,accessed 21.01.2014. (in Russian).
- 3D-printery [3D-printers]. Available at: http://habrahabr.ru/hub/3d-printers/ , accessed 17.01.2014. (in Russian).
- Batogov A. Sozdan 3D-printer, sposobnyj pechatat' uglevoloknom [3D-printer has been created, capable of printing by carbon fiber]. Available at: http://hi-news.ru/periferiya/sozdan-3d-printer-sposobnyj-pechatat-uglevoloknom.html , accessed 01.02.2014. (in Russian).
- Skljarov A.A. Prikladnye metody sinergeticheskogo sinteza ierarhicheskogo upravlenija avtonomnymi mobil'nymi robotami. Avtoreferat kand. diss. [Applied methods of synergetic synthesis of hierarchical autonomous mobile robots control: dissertation for the degree of candidate of technical sciences. Abstract of cand. diss.]. Taganrog, 2013. 20 p. (in Russian).
- Bezuglov A., Kolesnikov A., Kondratiev I., Vargas J. Synergetic Control Theory Approach for Solving Systems of Nonlinear Equations. Proc. of the 9th World Multi-Conference on Systemics, Cybernetics and Informatics, 2005, pp. 121-126.
- Glazunov V.F., Pikunov V.V., Repin A.A. [Synthesis method of synchronous motor control based on a synergistic approach]. Vestnik Ivanovskogo Gosudarstvennogo Energeticheskogo Universiteta – Vestnik of Ivanovo State Power Engineering University, 2005, no. 3. Available at: http://vestnik.ispu.ru/sites/vestnik.ispu.ru/files/publications/12-15.pdf , accessed 01.02.2014. (in Russian).
- Glazyrin A.S. [Sensorless control of an asynchronous electric drive with synergistic regulator]. Izvestiya Tomskogo politekhnicheskogo universiteta, 2012, vol. 321, no. 4: Power engineering, pp. 107-111. (in Russian).
- Kolesnikov A.L., Veselov P.E., Popov A.L., Kolesnikov Al.A., Kuzymenko A.A. Sinergeticheskoe upravlenie nelineynymi elektromekhanicheskimi sistemami [Synergetic Control by nonlinear Electromechanical Systems]. Moscow, ISPO-Servis Publ., 2000. 248 p. (in Russian).
- Santi E., Dougal R., Li D., Monti A., Prodduttur K. Synergetic Control for Power Electronics Applications: A Comparison with the Sliding Mode Approach. Journal of Circuits, Systems, and Computers, 2004, vol. 13, no. 4, pp. 737-760.
- Sinitsyn A.S., Kuz'menko A.A. [Using the principle of integral adaptation to increase the robustness of the synchronous generator excitation system]. Tekhnologii tekhnosfernoy bezopasnosti, 2013, no. 3 (49). Available at: http://academygps.ru/img/UNK/asit/ttb/2013-3/03-03-13.ttb.pdf , accessed 01.02.2014. (in Russian).
Publications with keywords: control design, computational fluid dynamics, rotordynamics, magnetic bearings, hemocompatibility, mechanical heart support system, ventricular assist device, heart failure
Publications with words: control design, computational fluid dynamics, rotordynamics, magnetic bearings, hemocompatibility, mechanical heart support system, ventricular assist device, heart failure
See also:
| Authors |
| Press-releases |
| Library |
| Conferences |
| About Project |
| Phone: +7 (915) 336-07-65 (строго: среда; пятница c 11-00 до 17-00) |
|
||||
| © 2003-2024 «Наука и образование» Перепечатка материалов журнала без согласования с редакцией запрещена Phone: +7 (915) 336-07-65 (строго: среда; пятница c 11-00 до 17-00) | |||||